For Healthcare Professionals
GSK is running a Phase 3 study to determine if belimumab is safe and effective in the treatment of systemic sclerosis associated interstitial lung disease (SSc-ILD).
Belimumab is a monoclonal antibody that has been approved for the treatment of systemic lupus erythematosus (SLE) and lupus nephritis that is now being studied for use in the treatment of systemic sclerosis associated interstitial lung disease (SSc-ILD).
BLISSc-ILD Study
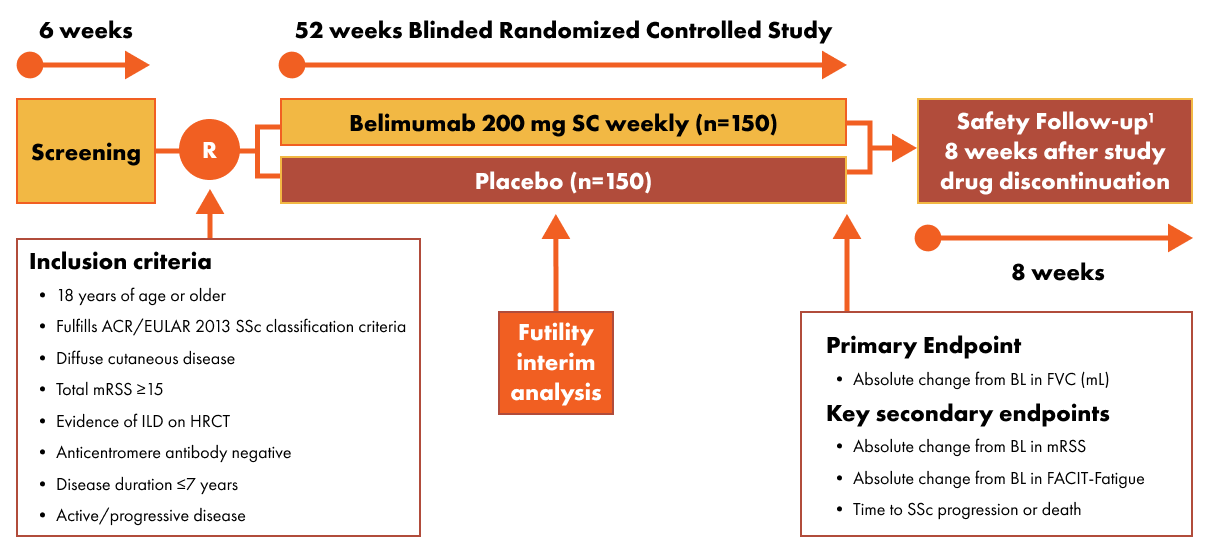
Patients diagnosed with SSc-ILD are invited to participate in a multicenter, randomized, double blind, placebo-controlled study to investigate the effectiveness and safety of belimumab versus placebo.
Participants will be randomized to one of two treatment arms for a total of 52 weeks:
- Treatment Arm 1: belimumab 200 mg SC weekly via syringe safety device for 52 weeks (n=150).
- Treatment Arm 2: matching placebo SC weekly via syringe safety device for 52 weeks (n=150).
Participants who complete the 52-week double-blind period, but do not enter the open-label extension study, will be required to return for a safety follow up visit 8 weeks after the last dose of study treatment.
Following the 52-week treatment period, participants may be eligible to join a separate open-label extension study where all participants will receive belimumab. Those who do not wish to, or are not eligible to join the open-label extension study, will be monitored for safety for at least 8 weeks following last dose of study treatment.
Systemic sclerosis is a rare chronic autoimmune connective tissue disease characterized by dysregulated immunity, vasculopathy, and tissue fibrosis with highly heterogenous clinical features, disease presentation, course, prognosis, and therapeutic response. Current treatment approaches for SSc and SSc-ILD generally include the off-label use of mycophenolate and methotrexate (for skin thickening only) as a first line therapy. Cyclophosphamide and rituximab are considered in patients not responding to mycophenolate; and azathioprine is used as maintenance after cyclophosphamide treatment or as an alternative to mycophenolate.
Belimumab is a monoclonal antibody that, through binding B lymphocyte stimulator (BLyS), has been shown to inhibit the survival of B cells and reduce differentiation into IgG-producing plasma cells. B cells have been reported to play a central role in the pathogenesis of SSc. For example, in patients with SSc, B cell depletion following treatment with rituximab has been shown to reduce skin thickening and slow down decline in lung function.1 Therefore, we hypothesize that belimumab, through neutralization of BLyS, will impact all 3 hallmarks of SSc (vasculopathy, inflammation, and fibrosis), which could lead to reduction in skin thickening, slowing down of lung function decline, and improved quality of life. Across previous systemic lupus erythematosus studies, treatment with belimumab plus standard of care was generally well tolerated.2
Inclusion Criteria
- Aged 18 years and older.
- Documented diagnosis of SSc as defined by the American College of Rheumatology / European League Against Rheumatism 2013 SSc classification criteria
- Diffuse cutaneous disease, with a total mRSS ≥15.
- Evidence of interstitial lung disease on centrally read screening HRCT.
- Evidence for active or progressive disease with a diagnosis SSc within the past 7 years
Exclusion Criteria
- FVC ≤45% of predicted, or a DLco (corrected for hemoglobin) ≤40% of predicted or requiring supplemental oxygen at screening.
- SSc renal crisis within 6 months prior to the first day of dosing (Day 1).
- Obstructive pulmonary disease (pre-bronchodilator FEV1/FVC <0.7)
- Significant emphysema on screening HRCT (extent of emphysema exceeds extent of ILD)
- Treatment with rituximab or cyclophosphamide (oral or intravenous) within 6 months prior to Day 1.
- Pulmonary arterial hypertension
- An active infection, or a history of serious infections
ACR = American College of Rheumatology; BL = baseline; EULAR = European League Against Rheumatism; FACIT = Functional Assessment of Chronic Illness Therapy; FVC = forced vital capacity; HRCT = high-resolution computed tomography; ILD = interstitial lung disease; mRSS = modified Rodnan skin score; SC = subcutaneously; SSc = systemic sclerosis.
1.Participants who complete the Week 52 visit and have not been classified as a treatment failure will be eligible to join a separate open-label extension study (Study 219855) and receive open-label treatment with belimumab 200 mg SC weekly.
Permitted concomitant immunosuppressive/immunomodulatory therapies:
Any one of the below listed agents, not taken in combination, are permitted, but not mandated:
- Mycophenolate mofetil (≤3000 mg/day) or equivalent mycophenolate sodium (≤2160 mg/day), provided participants have been on stable doses for at least 180 days, OR
- Methotrexate ≤25 mg/week, provided participants have been on stable doses for at least 90 days, OR
- Azathioprine ≤2.5 mg/kg/day, provided participants have been on stable doses for at least 90 days.
- In addition, participants who are taking oral corticosteroids (≤10 mg/day of prednisone or equivalent) are permitted in the study if the participant has been receiving a dose ≤10 mg/day for at least 30 days prior to Day 1.
In addition, participants who are taking oral corticosteroids (≤10 mg/day of prednisone or equivalent) are permitted in the study if the participant has been receiving a dose ≤10 mg/day for at least 30 days prior to Day 1.

Patient Commitment
The study will consist of:
- A screening period of up to 6 weeks
- Treatment period of up to 52 weeks with either belimumab or placebo alongside standard of care therapy
- Safety follow up period for approximately 8 weeks following last dose of belimumab (in either this parent study or the open-label extension study)
Total patient commitment may last approximately 15 months, with approximately 9 study visits. GSK is also offering travel support (travel arrangements or reimbursement for travel to and from the study site) for patients who are geographically distant from our study sites.
Where can I participate in the Study?
Enter your postal code below to find a study site near you.
Learn More About the Clinical Trials
Healthcare Professionals Only
If you are interested in learning more about how your patient(s) may participate, please use one of the options below to connect with a qualified healthcare professional or trial representative.
Schedule a call online:
Use the online calendar to schedule a time for a call that works best for you.
Call 1-877-934-5574 (Anytime Mon – Fri; 8am – 5pm CST)
Fill out the brief form below to receive additional study information:
Refer a Patient
Refer a Patient
Have a patient who might be a good fit?
Have your patients call 1-833-368-9645 and let the health care professional know you referred them. The health care professional can help answer any questions and walk them through initial pre-screening.
Have your patients visit blisscildstudy.com and complete the online pre-screener to see if they qualify. If they qualify and agree to be contacted, a member of the trial team will contact them for next steps.
Email your patients information: Clicking the button below will copy a short description and link to the clinical trial website to your clipboard, which can then be pasted into and edited in any secure messaging platform you use. This message also includes the pre-screening number.
References:
1. Ebata S, Yoshizaki A, Oba K, et al. Lancet Rheumatol. 2021;3(7):e489-e497.
2. Wallace DJ, Atsumi T, Daniels M, et al. Lupus. 2022;31(13):1649-1659.


